|
Introduction to Bacterial Stains
Most bacteria are difficult to see
under the bright field of a microscope. Bacteria are
almost colorless (composed primarily of water) and
therefore show little contrast with the suspension. To
visualize bacteria, either dyes or stains are used.
Since staining of bacterial cells is relatively fast,
inexpensive, and simple, it is the most commonly used
technique to visualize bacterial cells. Staining not
only makes bacteria more easily seen, but it allows
their morphology (e.g. size and shape) to be visualized
more easily. In some cases, specific stains can be used
to visualize certain structures (flagella, capsules,
endospores, etc). of bacterial cells.
There are several staining methods
that are used routinely with bacteria. These methods may
be classified as 1) simple and 2) differential.
Simple stains will react with all microbes in an
identical fashion. They are useful solely for increasing
contrast so that morphology, size, and arrangement of
organisms can be determined. Differential stains
give varying results depending on the organism being
treated. These results are often helpful in identifying
the microbe.
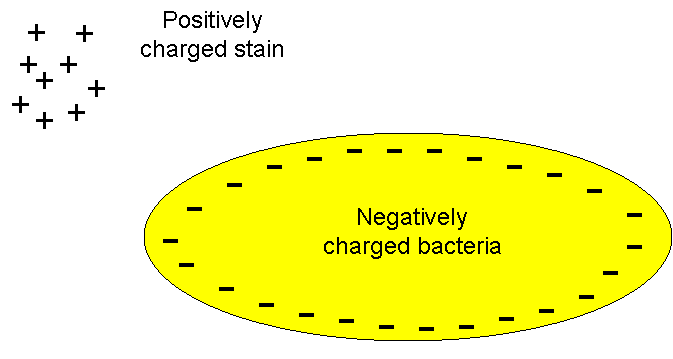
Commonly used microbiological
stains generally fall into one of two categories - basic
stains or acidic stains (although there are a few stains
such as India Ink) which are neutral). A basic dye
is a stain that is positively charged and will therefore
react with material that is negatively charged.
Bacterial cells have a slight negative charge will
therefore attract and bind with basic dyes. Some
examples of basic dyes are crystal violet, safranin,
basic fuchsin and methylene blue. Acid dyes are
negatively charged and are repelled by the bacterial
surface forming a deposit around the organism. They
stain the background and leave the microbe transparent.
Nigrosine and congo red are examples of acid dyes.
At first glance, the easiest way
to stain bacterial cells would appear to be simply
mixing the bacterial suspension with the dye and making
a wet mount of this mixture. Unfortunately, if you were
to try staining bacterial cells in this manner you would
find that there was too much background (unbound dye) to
allow for visualization of the cells. Therefore, you
need to remove the unbound dye. Simply washing off the
dye would result in removal of the cells along with the
excess dye. Therefore, you need a mechanism to fix
(adhere) the cells to the slide before staining to
allow for removal of excess dye while keeping the cells
on the slide. |
| A simple method is
that of air drying and heat fixing. The organisms are
heat fixed by passing an air-dried smear of the
organisms through the flame of a gas burner. The heat
coagulates the organisms' proteins causing the bacteria
to stick to the slide. Be very careful not to over heat
the organisms when fixing them to a slide. This distorts
the sample of the organisms. Keep in mind the analogy of
a fried egg. When you drop a raw egg onto a cold frying
pan, it has a certain shape. Start heating it and the
proteins (albumin) on the lower surface of the egg
precipitate and fix the egg to the pan. At this point
there has been a minimal distortion in the shape of the
egg as a whole; only a small percent of the proteins
have been precipitated. If you keep applying heat, the
shape of the entire egg will change and eventually it
will be reduced to charred remains. When you heat fix a
slide, you want to apply enough heat to precipitate the
proteins to allow the cells to stick to the slide but
not to drastically change the shape of the cells (or
reduce them to charred remains). |
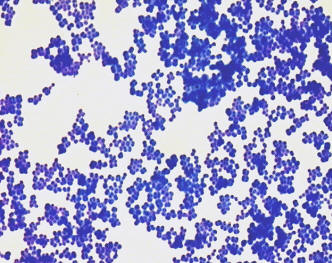
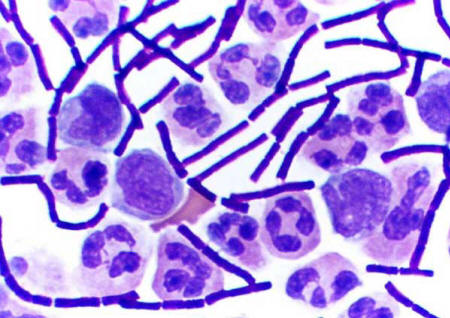
Figure 2.1 Cocci bacteria
stained blue |
|
|
Simple Stain
|
| Staining
is a technique that is used to enhance contrast in a
microscopic image. Staining can also be used to
highlight structures for viewing. The simple stain can
be used to determine cell shape, size, and arrangement.
The simple stain is a very simple staining procedure
involving only one stain. The most common stains (dyes)
used are methylene blue, Gram safranin, and Gram crystal
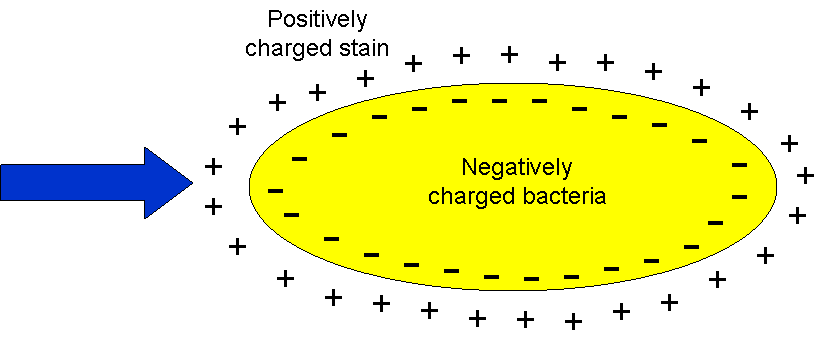
violet. Basic stains, such
as methylene blue, Gram safranin, or Gram crystal violet
are useful for staining most bacteria. These stains will
readily give up a OH- ion or accept a H+
ion, which leaves the stain positively charged. These
positively charged stains adhere readily to the cell
surface, since the surface of most bacteria are
negatively charged. |
Figure 2.2 Bacillus simple
stain |
PROCEDURE SIMPLE STAIN
1) Perform a bacterial smear of the given bacterial culture
2) Allow the smear to dry thoroughly.
3) Heat-fix the smear cautiously by passing the underside of the slide through the burner flame two or three times.Overheating can distort the cells.
4) Saturate the smear with basic dye and let sit for approximately 1 minute. You may use crystal violet, safranin, or methylene blue
5) Rinse the slide gently with water
6) Observe the slide under the microscope
 |
|
Figure 2.3
Bacteria cell before simple stain |
Figure 2.4
Bacteria cell after simple stain
|
|
|
|
|
|
Figure 2.5
Bacteria cell before simple stain
|
Figure 2.6
Bacteria cell after simple stain
|
|
|
VIRTUAL
LAB |
|
|
**Go to the site listed
below to access a virtual lab in which you will
perform a simple stain.
VIRTUAL SIMPLE STAIN
SKETCH 1
**Sketch a few of
the bacteria you have viewed in the microscope,
indicating any bacterial or background coloration
you observe either with colored markers (pens,
pencils, etc) or by labeling what colors you
observe. Be sure to include a few of each bacteria
if they have stained differently.
Click Here to Access a Chart to Produce Your
Sketches (MS WORD)
Click Here to
Access a Chart to Produce Your Sketches (PDF)
|
|
|
Endospore Stain
The endospore stain is a differential
stain used to visualize bacterial endospores. Endospores are
formed by some bacteria, such as
Bacillus. By forming spores, bacteria can survive in
hostile conditions. Spores are resistant to heat, dessication,
chemicals, and radiation. Bacteria can form endospores in
approximately 6 to 8 hours after being exposed to adverse
conditions. The normally growing cell that forms the endospore
is called a vegetative cell. Spores are metabolically inactive
and dehydrated. They can remain viable for thousands of
years. When spores are exposed to favorable conditions, they can
germinate into a vegetative cell within 90 minutes.
|
| Endospores can form within different areas
of the vegetative cell. They can be central, subterminal, or
terminal. Central endospores are located within the middle of
the vegetative cell. Terminal endospores are located at the end
of the vegetative cell. Subterminal endospores are located
between the middle and the end of the cell. Endospores can also
be larger or smaller in diameter than the vegetative cell. Those
that are larger in diameter will produce an area of "swelling"
in the vegetative cell. These endospore characteristics are
consistent within the spore-forming species and can be used to
identify the organism.
Because of their tough protein coats made
of keratin, spores are highly resistant to normal staining
procedures. The primary stain in the endospore stain procedure,
malachite green, is driven into the cells with heat and readily
adheres to the endospore. Since
malachite green is water-soluble and does not adhere well to the
cell, and since the vegetative cells have been disrupted by
heat, the malachite green rinses easily from the vegetative
cells, allowing them to readily take up the counterstain. This
allows the endospores to be visible with the malachite
green stain and the vegetative cells to be visible with
the counterstain.
|
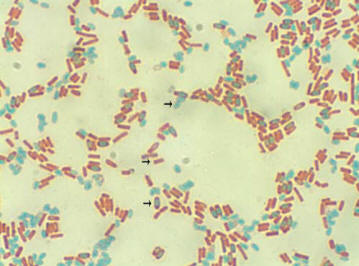
Figure 2.7
Bacilli endospores stained green and vegetative
cells stained red |
PROCEDURE ENDOSPORE STAIN
1) Perform a bacterial smear of Bacillus or the organism you are given to stain
2) Saturate
the smear with malachite green
3) Heat the slide gently over a Bunsen burner for 5 minutes
4) Rinse the slide gently with water
5) Counterstain
with safranin for 2 minutes
6) Rinse the slide gently with water
7) Observe the slide under the microscope. Endospores
will stain green. Vegetative cells will stain red
|
|
|
|
|
|
Capsule Stain
The capsule stain employs an acidic stain
and a basic stain to detect capsule production. Capsules are
formed by organisms such as
Klebsiella pneumoniae. Most capsules are composed of
polysaccharides, but some are composed of polypeptides. The
capsule differs from the slime layer that most bacterial cells
produce in that it is a thick, detectable, discrete layer
outside the cell wall. Some capsules have well-defined
boundaries, and some have fuzzy, trailing edges. Capsules
protect bacteria from the phagocytic action of leukocytes and
allow pathogens to invade the body. If a pathogen loses its
ability to form capsules, it can become avirulent.
|
| Bacterial capsules are non-ionic, so
neither acidic nor basic stains will adhere to their surfaces.
Therefore, the best way to visualize them is to stain the
background using an acidic stain and to stain the cell itself
using a basic stain. We will use India ink and Gram crystal violet.
This leaves the capsule as a clear halo surrounding a purple
cell in a field of black.
The medium in which the culture is grown
as well as the temperature at which it is grown and the age of
the culture will affect capsule formation. Older cultures are
more likely to exhibit capsule production. When performing a
capsule stain on your unknown, be sure the culture you take your
sample from is at least five days old.
The ability
to produce a capsule is an inherited property of the
organism, but the capsule is not an absolutely essential
cellular component. Capsules help many pathogenic and
normal flora bacteria to initially resist phagocytosis
by the host's phagocytic cells. In soil and water,
capsules help prevent bacteria from being engulfed by
protozoans. Capsules also help many bacteria to adhere
to surfaces and thus resist flushing. It also enables
many bacteria to form biofilms. A
biofilm
consists layers of bacterial populations adhering to
host cells and embedded in a common capsular mass.
|
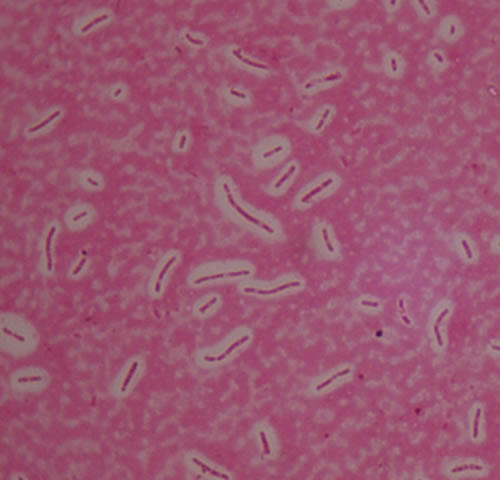
Figure 2.8
Bacilli capsule stain
|
PPROCEDURE CAPSULE STAIN
1) Place a single drop
of India ink on a microscope slide
2) Add the organism to be
stained and mix into the drop of India ink
3) Spread the mixture on the slide to a thin
layer
4) Allow the film to air
dry. DO NOT heat or blot dry. Heat will melt
the capsule
5) Saturate the slide
with crystal violet for 1 minute
6) Rinse the slide gently
with water
7) Allow the slide to air
dry
8) Observe the slide under the
microscope. The background will be dark. The
bacterial cells will be stained purple. The capsule
(if present) will appear clear against the dark
background
|
|
|
|
|
|
Acid Fast Stain
The acid-fast stain is a differential
stain used to identify acid-fast organisms such as members of
the genus Mycobacterium. Acid-fast organisms are characterized by
wax-like, nearly impermeable cell walls; they contain mycolic
acid and large amounts of fatty acids, waxes, and complex
lipids. Acid-fast organisms are highly resistant to
disinfectants and dry conditions.
|
|
Because the cell wall is so resistant to
most compounds, acid-fast organisms require a special staining
technique. The primary stain used in acid-fast staining, carbolfuchsin, is lipid-soluble and contains phenol, which helps
the stain penetrate the cell wall. This is further assisted by
the addition of heat. The smear is then rinsed with a very
strong decolorizer, which strips the stain from all
non-acid-fast cells but does not permeate the cell wall of
acid-fast organisms. The decolorized non-acid-fast cells then
take up the counterstain.
The acid-fast stain
is an especially important test for the genus
Mycobacterium. There are two distinct pathogens in
this group: M. tuberculosis, the causative
organism of tuberculosis, and M. leprae, the
causative agent of leprosy. |
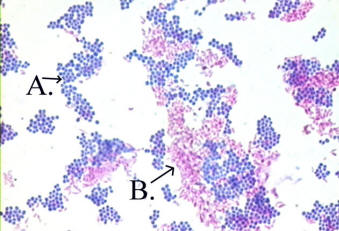
Figure 2.9
Acid Fast stain
(A) non-acid fast bacteria, (B) acid fast bacteria
|
PPROCEDURE ACID FAST
STAIN
1) Perform a bacterial smear
2) Flood
the smear with carbolfuchsin
3) Heat the slide gently over the Bunsen
burner for 5 minutes
4) Rinse the slide gently with water
5) Decolorize the slide with acid-alcohol
until the rinsate runs clear
6) Rinse the slide gently with water
7) Counterstain with methylene blue for
2 minutes
8) Rinse the slide gently with water
9) Observe the slide under the
microscope. Acid-fast cells will stain fuchsia
(pink or red). Non-acid-fast cells will stain blue
|
|
|
|
|
Negative Stain
|
| The negative stain is particularly useful
for determining cell size and arrangement. It can also be used
to stain cells that are too delicate to be heat-fixed. We will use nigrosin as our negative stain.
Nigrosin is an acidic stain. This means
that the stain readily gives up a hydrogen ion and becomes
negatively charged. Since the surface of most bacterial cells
is negatively charged, the cell surface repels the stain. The
glass of the slide will stain, but the bacterial cells will not.
The bacteria will show up as clear spots against a dark
background.
Negative staining is an
excellent way to determine an organismís cellular morphology.
Since the cells themselves are not
stained, their morphology is not distorted in any way. The
nigrosin provides a dark
background against which the shapes of the unstained cells are
clearly visible. This method provides a high
degree of contrast not available in most other staining
procedures.
|
Figure 2.10
Negative Stain
|
PPROCEDURE NEGATIVE STAIN
1) Place a single drop
of nigrosin on a microscope slide
2) Mix a sample of your
organism to stain into the drop of nigrosin
3) Spread the sample on
the slide into a thin layer
4) Allow the
film to air dry
5) Observe the slide
under the microscope. The bacteria will be clear and
the background will stain dark
|
|
|
|
|
Gram
Stain
The Gram stain is the most important
staining procedure in microbiology. It is used to differentiate
between gram positive organisms and gram negative organisms.
Hence, it is a differential
stain. Gram negative and gram positive organisms
are distinguished from each other by differences in their cell
walls. These differences affect many aspects of the cell,
including the way the cell takes up and retains stains.
Gram positive cells take up crystal
violet, which is then fixed in the cell with iodine mordant.
This forms a crystal-violet iodine complex which remains in the
cell even after decolorizing. It is thought that this happens
because the cell walls of gram positive organisms include a
thick layer of protein-sugar complexes called peptidoglycans.
This layer makes up 60-90% of the gram positive cell wall.
Decolorizing the cell causes this thick cell wall to dehydrate
and shrink, which closes the pores in the cell wall and prevents
the stain from exiting the cell. At the end of the gram
staining procedure, gram positive cells will be stained a
purplish-blue color.
|
Gram negative cells also take up crystal
violet, and the iodine forms a crystal violet-iodine complex in
the cells as it did in the gram positive cells. However, the
cell walls of gram negative organisms do not retain this complex
when decolorized. Peptidoglycans are present in the cell walls
of gram negative organisms, but they only comprise 10-20% of the
cell wall. Gram negative cells also have an outer layer which
gram positive organisms do not have; this layer is made up of
lipids, polysaccharides, and proteins. Exposing gram negative
cells to the decolorizer dissolves the lipids in the cell walls,
which allows the crystal violet-iodine complex to leach out of
the cells. This allows the cells to subsequently be stained with
safranin. At the end of the gram staining procedure, gram
negative cells will be stained a reddish-pink color.
Often, detecting the presence of
microorganisms and determining whether an infection
is caused by an organism that is Gram-positive or
Gram-negative will be sufficient to allow a doctor
to prescribe treatment with an appropriate
antibiotic while waiting for more specific tests,
such as a culture, to be completed.
|
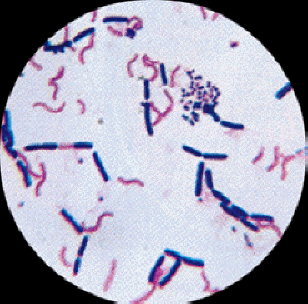
Figure 2.11
Gram Stain
|
PROCEDURE
1) Perform a bacterial
smear of your given culture
2) Saturate
the smear with crystal violet for 1 minute
3) Rinse the slide gently
with water
4) Saturate the smear
with iodine for 1 minute
5) Rinse the slide gently
with water
6) Decolorize with Gram
decolorizer (acetone/alcohol) for 3-5 seconds
7) Rinse the slide gently
with water
8) Counterstain with safranin for
1 minute
9) Rinse the slide gently
with water
10) Observe the slide
under the microscope. Gram positive bacteria will
stain purple. Gram negative bacteria will stain
red/pink
|
|
|
|
QUESTIONS |
**Answer the
questions on bacterial staining given below
1) While performing the stains, why was it important to
not let the slide get too hot from the bunsen burners?
2) How does a simple stain differ from a negative stain?
3) What is the difference between a simple stain and a
differential stain?
4) What does "fixing" mean when performing bacterial
stains?
5) How are positively charged dyes able to adhere to
bacteria?
6) What survival advantage is gained in bacteria that
produce capsules?
7) How long can endospores survive?
8) What survival advantage do acid fast bacteria have
over other non-acid fast bacteria?
9) What variety of stain is negatively charged rather
than positively charged?
10) List some differences in gram negative and gram
positive bacterial cell walls.
Click Here for an MS WORD Version of the Bacterial Stain
Questions
Click Here for a PDF
Version of the Bacterial Stain Questions
|
|